Home » Applications » Expanding Research on Solar Energy Applications
Expanding Research on Solar Energy Applications
The application fields of solar energy are very wide, covering many fields such as the photonic industry, new energy photothermal projects, solar detection, climate action, nuclear fusion research, and new energy vehicles. With the continuous innovation and advancement of technology, the application scenarios of solar energy will be more abundant, making greater contributions to the sustainable development of human society. Some interesting things are happening in universities around the world that may eventually find their way into solar cell production. Here are a few examples.
Australian National University - Plasmonic Particles
 Kylie Catchpole, from the Australian National University (ANU) in Canberra, discovered that silver nanoparticles 100 nanometers (billionths of a meter) in size that are deposited on the "surface" of thin film silicon solar cells do not reflect light that has fallen on them as one would expect. Instead, the light is bounced back and forth within the cell allowing longer wavelengths to be absorbed due to the plasmonic effect. (The plasmonic effect is the oscillation of electrons in a solid stimulated by incident light. Resonance occurs when the frequency of the light photons matches the natural frequency of the surface electrons.) The light comes in, gets scattered by the nanoparticle, and then is absorbed by the solar cell. The light is not absorbed by the silver nanoparticle. The nanoparticles on the surface do not affect the workings of the solar cell below, they just increase the absorption.
Kylie Catchpole, from the Australian National University (ANU) in Canberra, discovered that silver nanoparticles 100 nanometers (billionths of a meter) in size that are deposited on the "surface" of thin film silicon solar cells do not reflect light that has fallen on them as one would expect. Instead, the light is bounced back and forth within the cell allowing longer wavelengths to be absorbed due to the plasmonic effect. (The plasmonic effect is the oscillation of electrons in a solid stimulated by incident light. Resonance occurs when the frequency of the light photons matches the natural frequency of the surface electrons.) The light comes in, gets scattered by the nanoparticle, and then is absorbed by the solar cell. The light is not absorbed by the silver nanoparticle. The nanoparticles on the surface do not affect the workings of the solar cell below, they just increase the absorption.
The sketch at the right shows long wavelengths of light striking small silver metal nanoparticles on the surface of thin film silicon only several microns (1 micron = one-millionth of a meter) thick. The long wavelengths are then absorbed by the silicon below as opposed to passing through the silicon as is the case for normal thin film silicon. These experimental silver-coated solar cells produce 30% more electricity than conventional thin-film silicon cells because of the absorption of the long wavelengths of light. Processes now have to be developed to be able to mass produce the experimental cells commercially. Commercial solar cells using this technology are expected in about three years.
National Renewable Energy Laboratory (NREL) - Plasmonic Particles
The NREL in Boulder, Colorado is using Quantum Dots (QDs) to generate more than one electron-hole pair for every photon absorbed. However, the NREL's QDs are "inside" the solar cell as opposed to being on the surface (as at ANU above). No solar cells produced before December 2011 have quantum efficiencies greater than 100 percent. Quantum efficiency (not to be confused with solar cell efficiency) per the NREL is the “ratio of collected charge carriers (electrons or electron holes) to incident photons”. In layman’s terms - it’s the ratio of the number of electrons produced in a solar cell to the number of the sun's photons hitting the cell.
Researchers from the NREL have demonstrated quantum efficiencies of 114 percent in solar cells “excited” from photons from the high-energy region of the solar spectrum. That is from the near ultraviolet through the visible light spectrum, 0.3 to 0.7 micrometers (millionths of a meter). See the solar radiation chart above. Energy is always conserved. The extra electrons come from the extra energy left over after the initial photon-electron collision. Light photons with wavelengths below 0.7 micrometers do not have enough energy to dislodge more than one electron.
NREL achieved this result with a layered quantum dot "experimental cell" composed of a surface of anti-reflective glass, a thin layer of semiconductor zinc oxide “textured” at the nano level, a QD layer of lead selenide doped with ethanethiol (a bonding agent) and hydrazine (a deposition stabilizer), and a thin layer of gold for the collector electrode.
This process, which creates more than one electron-hole pair from a single photon, is called "multiple exciton generation" (MEG) by NREL. However, it should be emphasized that the research into Quantum Dots is at a very basic stage of demonstrating scientific principles. No one at this time has made a pre-production Quantum Dot solar cell. Production of solar cells using Quantum Dots is thought to be about 10 years into the future.
California Institute of Technology - Nanoparticle Wires
Harry Atwater and fellow researchers at Caltech have developed arrays of a minute silicon micro wires - 1 micron in diameter and up to 100 microns high - that are embedded in a thin transparent rubbery polymer that absorbs enough sunlight to have a potential efficiency of 15 to 20%, as good as the best crystalline cells of today. See the Solar Efficiency Limits page.
 While these arrays have the thickness of a conventional crystalline solar cell, their semiconductor volume is equivalent to that of a two-micron thick film. These solar cells use only about two percent as much silicon as their big brothers. The transparent polymer contains reflective nanoparticles of aluminum that shuttle light back and forth in the cell until it is finally absorbed by a tiny wire of silicon. The experimental arrays are on the order of a few square centimeters in size. To be commercially viable, these cells have to be scaled up by a factor of 100 times or more. One feature of this technology is that the final solar cell is very flexible which opens up new solar BIPV markets as shown above in the CIGS section.
While these arrays have the thickness of a conventional crystalline solar cell, their semiconductor volume is equivalent to that of a two-micron thick film. These solar cells use only about two percent as much silicon as their big brothers. The transparent polymer contains reflective nanoparticles of aluminum that shuttle light back and forth in the cell until it is finally absorbed by a tiny wire of silicon. The experimental arrays are on the order of a few square centimeters in size. To be commercially viable, these cells have to be scaled up by a factor of 100 times or more. One feature of this technology is that the final solar cell is very flexible which opens up new solar BIPV markets as shown above in the CIGS section.
Stanford University - Photon Enhanced Thermionic Emission (PETE)
A conventional thermionic converter (used on satellites) is driven solely by intense heat (1,500°C) and converts thermal energy into electricity. The converter consists of two electrodes separated by a vacuum. When the cathode is heated to a high temperature, electrons become excited, jump across the thin vacuum to the relatively cold anode, and drive a current through an external circuit back to the cathode.
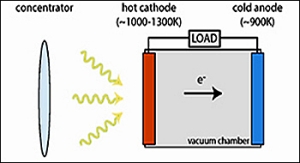 The Stanford Photon Enhanced Thermionic Emission (PETE) prototype uses concentrated sunlight as its source of energy and in a two-step process uses both the sun's photon energy and its heat to excite the cathode electrons to jump across the vacuum to the collector anode. The cathode emitter is a semiconductor material rather than a metal electrode. First, the sunlight's photons partially excite the electrons in the cathode semiconductor (similar to a silicon PV cell) so that in step two the remaining heat energy necessary for emission is lower than that for a standard thermionic converter (but not as low as a regular solar cell). The surface of the cathode on the vacuum side is texturized to increase emissions. PETE converts about 25% of the sunlight's energy into electricity.at 200°C and higher efficiencies at higher temperatures, i.e. 45% at 1000°C.
The Stanford Photon Enhanced Thermionic Emission (PETE) prototype uses concentrated sunlight as its source of energy and in a two-step process uses both the sun's photon energy and its heat to excite the cathode electrons to jump across the vacuum to the collector anode. The cathode emitter is a semiconductor material rather than a metal electrode. First, the sunlight's photons partially excite the electrons in the cathode semiconductor (similar to a silicon PV cell) so that in step two the remaining heat energy necessary for emission is lower than that for a standard thermionic converter (but not as low as a regular solar cell). The surface of the cathode on the vacuum side is texturized to increase emissions. PETE converts about 25% of the sunlight's energy into electricity.at 200°C and higher efficiencies at higher temperatures, i.e. 45% at 1000°C.
However, not all the heat is consumed, the surplus heat can be used to feed an auxiliary heat engine. Coupling a PETE device with a thermal heat engine such as a parabolic solar trough system, which already has a steam turbine engine (see Parabolic Trough Systems), the total energy efficiency could be in the 50% to 60% range - a major improvement over current solar technologies. See the Solar Efficiency Limits page. This technology would not be used on rooftop systems because of the extreme temperatures. But think of a solar front end concentrating the sunlight, a PETE conversion station, and a back end parabolic trough/steam turbine generator.
This type of hybrid solar system could be used by utilities to generate grid electricity. That is the "vision". A lot of work needs to be done to get from today's laboratory setup to a production product in the field. A competitive product is probably 8 to 10 years away.
MIT - Can Solar Energy Be Economically Stored?
 Stored power has two major issues that have been holding back widespread acceptance of the technology: 1) the initial capital equipment cost is expensive relative to other alternatives, and 2) solar power is a daytime phenomenon. In the first case, the cost of PV solar is coming down rapidly and is expected to reach "grid parity" in a few years. In the second case, storage of solar power in batteries is very expensive and not enough capacity exists, the same as other alternatives. Daniel Nocera, Professor of Chemistry and Energy at MIT, and assistant, Matthew Kanan Postdoctoral Fellow, have made a scientific discovery that may lead to a cheap way to store solar energy in the future.Water is H2O - two hydrogen atoms and an oxygen atom. Scientists have known for a long time how to use electricity to split water into hydrogen and oxygen, which is called electrolysis. The discovery is a way of doing what existing electrolyzers do, but much more simply and efficiently. The key component in the process is a new catalyst which consists of cobalt metal, phosphate, and an electrode. Running a small electric current through the water, the water's oxygen atoms bubble up, leaving pure hydrogen in the water. Another separate catalyst recovers the hydrogen gas. Pure hydrogen, is an excellent way to store energy.
Stored power has two major issues that have been holding back widespread acceptance of the technology: 1) the initial capital equipment cost is expensive relative to other alternatives, and 2) solar power is a daytime phenomenon. In the first case, the cost of PV solar is coming down rapidly and is expected to reach "grid parity" in a few years. In the second case, storage of solar power in batteries is very expensive and not enough capacity exists, the same as other alternatives. Daniel Nocera, Professor of Chemistry and Energy at MIT, and assistant, Matthew Kanan Postdoctoral Fellow, have made a scientific discovery that may lead to a cheap way to store solar energy in the future.Water is H2O - two hydrogen atoms and an oxygen atom. Scientists have known for a long time how to use electricity to split water into hydrogen and oxygen, which is called electrolysis. The discovery is a way of doing what existing electrolyzers do, but much more simply and efficiently. The key component in the process is a new catalyst which consists of cobalt metal, phosphate, and an electrode. Running a small electric current through the water, the water's oxygen atoms bubble up, leaving pure hydrogen in the water. Another separate catalyst recovers the hydrogen gas. Pure hydrogen, is an excellent way to store energy.
The oxygen and hydrogen gases can be stored in separate containers. At a later time, the oxygen and hydrogen gases can then be fed into a fuel cell, creating carbon-free electricity. (Fuel cells combine two gases, usually hydrogen and oxygen, to make electricity.) In the MIT lab, electricity came from the grid, but the electricity could come from solar panels, or for that matter, from wind turbines or hydropower. This system imitates the water-splitting reaction that occurs in nature which is called photosynthesis (carbon dioxide + water + light => stored sugar + waste oxygen).
 Dan Nocera's vision for the future is that each residential house will have a PV solar panel system on the roof. During the day the sun will generate electricity and the excess, that is not currently used for heat, air conditioning, etc., will be converted to hydrogen and oxygen gas using only ordinary water and stored in local tanks. When needed, the hydrogen and oxygen gas will be fed into a fuel cell to generate electricity at night or on cloudy days. The electricity could be used for home appliances or to charge an electric car or anything else. The fuel cell by-product is again water which would be fed back into the water tank. Most likely a connection to the grid would be maintained for backup purposes. This vision is probably ten-plus years away. Still, scientists need to have a road map to the future to guide them in their day-to-day activities along the way. Dr. Nocera has founded a new start-up company in the Boston area, Sun Catalytix Corporation, thanks to a $700,000 seed investment led by Polaris Ventures. That might sound like a small sum, but Nocera has been an epic center of buzz ever since he first published the research behind Sun Catalytix.
Dan Nocera's vision for the future is that each residential house will have a PV solar panel system on the roof. During the day the sun will generate electricity and the excess, that is not currently used for heat, air conditioning, etc., will be converted to hydrogen and oxygen gas using only ordinary water and stored in local tanks. When needed, the hydrogen and oxygen gas will be fed into a fuel cell to generate electricity at night or on cloudy days. The electricity could be used for home appliances or to charge an electric car or anything else. The fuel cell by-product is again water which would be fed back into the water tank. Most likely a connection to the grid would be maintained for backup purposes. This vision is probably ten-plus years away. Still, scientists need to have a road map to the future to guide them in their day-to-day activities along the way. Dr. Nocera has founded a new start-up company in the Boston area, Sun Catalytix Corporation, thanks to a $700,000 seed investment led by Polaris Ventures. That might sound like a small sum, but Nocera has been an epic center of buzz ever since he first published the research behind Sun Catalytix.
There are still several major problems to be solved: Sun Catalytix needs to replace the platinum used in the "other electrode" in Nocera's hydrogen process with something cheaper. (Platinum is not cheap.) Also, the large-scale processes for manufacturing need to be worked out. Sun Catalytix recently received a contract for over $4 million in federal funding from the US Department of Energy (DOE). DOE selected Sun Catalytix as one of only 37 recipients from over 3,600 applicants for the first round of funding for transformational energy technologies. In October 2010 Sun Catalytix raised $9.7 million in a second round of financing led by Tata Limited.
There is competition, Nanoptek Corporation, also in the Boston suburbs, is pursuing its own catalytic, water-splitting process, and has raised $4.7 million in financing. For now, both companies are in stealth mode, refusing press inquiries. Their goals are obvious: A cheap way to get energy from the sun, just like plants do.
Space Based Solar Power (SBSP)
The ultimate solar power station is in outer space. As shown in the sketch at the far right, there are energy losses as the sun's rays are reflected from the atmosphere, clouds, dust, the day-night cycle and seasonal changes. Only about 50% of the sun's energy reaches the earth and then only 15% to 25% of that reaches the grid.
 As shown on the right-hand side of the sketch, a Space Based Solar Power (SBSP) system would collect solar energy by a satellite 24 hours a day, convert it to microwaves, and transmit the microwave radiation to Earth where it would be captured by a very large ground antenna and converted into usable electricity on the grid. The energy efficiency from the space station to the grid would be about 50%, roughly the same as fossil fuel electrical plants.
As shown on the right-hand side of the sketch, a Space Based Solar Power (SBSP) system would collect solar energy by a satellite 24 hours a day, convert it to microwaves, and transmit the microwave radiation to Earth where it would be captured by a very large ground antenna and converted into usable electricity on the grid. The energy efficiency from the space station to the grid would be about 50%, roughly the same as fossil fuel electrical plants.
The SBSP concept was first published in November 1968 by Dr. Peter Glaser. In 1973 he was granted a U.S. patent for his method of transmitting power from a satellite using microwaves sent from a large antenna to a combination rectifier-antenna, now called a "rectenna" on the ground.
NASA began to study the concept in 1974. They found that the concept had several major problems - the huge expense of putting the solar satellite in orbit requiring hundreds of space trips and the lack of experience in space for projects of this scale. The proposal showed enough promise to merit further research. Research has continued to this day.
 There have been enormous improvements in solar panel efficiency. Research has continued to this day. There have been enormous improvements in solar panel efficiency reducing the size of the panel array in space. (See an artist's sketch of a proposed satellite system at the left.) Rocketry has also improved, but not sufficiently enough that "hundreds of transport trips" can be undertaken cost-effectively at this time. Satellite transportation costs remain the biggest obstacle to this technology being implemented. Many noted scientists believe that if the US "concentrated" on this effort, the costs of transporting the spacecraft materials to space would come down and the whole project would be cost-competitive with fossil fuels.
There have been enormous improvements in solar panel efficiency. Research has continued to this day. There have been enormous improvements in solar panel efficiency reducing the size of the panel array in space. (See an artist's sketch of a proposed satellite system at the left.) Rocketry has also improved, but not sufficiently enough that "hundreds of transport trips" can be undertaken cost-effectively at this time. Satellite transportation costs remain the biggest obstacle to this technology being implemented. Many noted scientists believe that if the US "concentrated" on this effort, the costs of transporting the spacecraft materials to space would come down and the whole project would be cost-competitive with fossil fuels.
As we come to the later part of the 21st century and with the availability of fossil fuels declining resulting in extremely high fuel prices, many scientists believe SBSP will be seen as the ultimate answer. The real question is "Will we by then be ready to exploit this endless source of cheap electrical power?" For a more detailed discussion of SBSP see the Solar High Brochure by Dr. Philip Chapman former NASA Astronaut, Physics Professor at MIT, and Employee of Dr. Peter Glaser.
Ohio University has initiated a "SunSat Design Competition" which is "an international competition intended to accelerate the design, manufacture, launch, and operation of the next generation satellites that will collect energy in space and deliver it to earth as electricity. The Mission of the SunSat Design Initiative is to move space solar power out of the research labs and onto the public agenda. This will be done by virtual storytelling and networking on a global basis, explaining what space solar power is and how and why it will become the ultimate renewable energy resource for Planet Earth." See the Ohio University SunSat Design Competition.
Australian National University - Plasmonic Particles
 Kylie Catchpole, from the Australian National University (ANU) in Canberra, discovered that silver nanoparticles 100 nanometers (billionths of a meter) in size that are deposited on the "surface" of thin film silicon solar cells do not reflect light that has fallen on them as one would expect. Instead, the light is bounced back and forth within the cell allowing longer wavelengths to be absorbed due to the plasmonic effect. (The plasmonic effect is the oscillation of electrons in a solid stimulated by incident light. Resonance occurs when the frequency of the light photons matches the natural frequency of the surface electrons.) The light comes in, gets scattered by the nanoparticle, and then is absorbed by the solar cell. The light is not absorbed by the silver nanoparticle. The nanoparticles on the surface do not affect the workings of the solar cell below, they just increase the absorption.
Kylie Catchpole, from the Australian National University (ANU) in Canberra, discovered that silver nanoparticles 100 nanometers (billionths of a meter) in size that are deposited on the "surface" of thin film silicon solar cells do not reflect light that has fallen on them as one would expect. Instead, the light is bounced back and forth within the cell allowing longer wavelengths to be absorbed due to the plasmonic effect. (The plasmonic effect is the oscillation of electrons in a solid stimulated by incident light. Resonance occurs when the frequency of the light photons matches the natural frequency of the surface electrons.) The light comes in, gets scattered by the nanoparticle, and then is absorbed by the solar cell. The light is not absorbed by the silver nanoparticle. The nanoparticles on the surface do not affect the workings of the solar cell below, they just increase the absorption.The sketch at the right shows long wavelengths of light striking small silver metal nanoparticles on the surface of thin film silicon only several microns (1 micron = one-millionth of a meter) thick. The long wavelengths are then absorbed by the silicon below as opposed to passing through the silicon as is the case for normal thin film silicon. These experimental silver-coated solar cells produce 30% more electricity than conventional thin-film silicon cells because of the absorption of the long wavelengths of light. Processes now have to be developed to be able to mass produce the experimental cells commercially. Commercial solar cells using this technology are expected in about three years.
National Renewable Energy Laboratory (NREL) - Plasmonic Particles
The NREL in Boulder, Colorado is using Quantum Dots (QDs) to generate more than one electron-hole pair for every photon absorbed. However, the NREL's QDs are "inside" the solar cell as opposed to being on the surface (as at ANU above). No solar cells produced before December 2011 have quantum efficiencies greater than 100 percent. Quantum efficiency (not to be confused with solar cell efficiency) per the NREL is the “ratio of collected charge carriers (electrons or electron holes) to incident photons”. In layman’s terms - it’s the ratio of the number of electrons produced in a solar cell to the number of the sun's photons hitting the cell.
Researchers from the NREL have demonstrated quantum efficiencies of 114 percent in solar cells “excited” from photons from the high-energy region of the solar spectrum. That is from the near ultraviolet through the visible light spectrum, 0.3 to 0.7 micrometers (millionths of a meter). See the solar radiation chart above. Energy is always conserved. The extra electrons come from the extra energy left over after the initial photon-electron collision. Light photons with wavelengths below 0.7 micrometers do not have enough energy to dislodge more than one electron.
NREL achieved this result with a layered quantum dot "experimental cell" composed of a surface of anti-reflective glass, a thin layer of semiconductor zinc oxide “textured” at the nano level, a QD layer of lead selenide doped with ethanethiol (a bonding agent) and hydrazine (a deposition stabilizer), and a thin layer of gold for the collector electrode.
This process, which creates more than one electron-hole pair from a single photon, is called "multiple exciton generation" (MEG) by NREL. However, it should be emphasized that the research into Quantum Dots is at a very basic stage of demonstrating scientific principles. No one at this time has made a pre-production Quantum Dot solar cell. Production of solar cells using Quantum Dots is thought to be about 10 years into the future.
California Institute of Technology - Nanoparticle Wires
Harry Atwater and fellow researchers at Caltech have developed arrays of a minute silicon micro wires - 1 micron in diameter and up to 100 microns high - that are embedded in a thin transparent rubbery polymer that absorbs enough sunlight to have a potential efficiency of 15 to 20%, as good as the best crystalline cells of today. See the Solar Efficiency Limits page.
Stanford University - Photon Enhanced Thermionic Emission (PETE)
A conventional thermionic converter (used on satellites) is driven solely by intense heat (1,500°C) and converts thermal energy into electricity. The converter consists of two electrodes separated by a vacuum. When the cathode is heated to a high temperature, electrons become excited, jump across the thin vacuum to the relatively cold anode, and drive a current through an external circuit back to the cathode.
 The Stanford Photon Enhanced Thermionic Emission (PETE) prototype uses concentrated sunlight as its source of energy and in a two-step process uses both the sun's photon energy and its heat to excite the cathode electrons to jump across the vacuum to the collector anode. The cathode emitter is a semiconductor material rather than a metal electrode. First, the sunlight's photons partially excite the electrons in the cathode semiconductor (similar to a silicon PV cell) so that in step two the remaining heat energy necessary for emission is lower than that for a standard thermionic converter (but not as low as a regular solar cell). The surface of the cathode on the vacuum side is texturized to increase emissions. PETE converts about 25% of the sunlight's energy into electricity.at 200°C and higher efficiencies at higher temperatures, i.e. 45% at 1000°C.
The Stanford Photon Enhanced Thermionic Emission (PETE) prototype uses concentrated sunlight as its source of energy and in a two-step process uses both the sun's photon energy and its heat to excite the cathode electrons to jump across the vacuum to the collector anode. The cathode emitter is a semiconductor material rather than a metal electrode. First, the sunlight's photons partially excite the electrons in the cathode semiconductor (similar to a silicon PV cell) so that in step two the remaining heat energy necessary for emission is lower than that for a standard thermionic converter (but not as low as a regular solar cell). The surface of the cathode on the vacuum side is texturized to increase emissions. PETE converts about 25% of the sunlight's energy into electricity.at 200°C and higher efficiencies at higher temperatures, i.e. 45% at 1000°C.However, not all the heat is consumed, the surplus heat can be used to feed an auxiliary heat engine. Coupling a PETE device with a thermal heat engine such as a parabolic solar trough system, which already has a steam turbine engine (see Parabolic Trough Systems), the total energy efficiency could be in the 50% to 60% range - a major improvement over current solar technologies. See the Solar Efficiency Limits page. This technology would not be used on rooftop systems because of the extreme temperatures. But think of a solar front end concentrating the sunlight, a PETE conversion station, and a back end parabolic trough/steam turbine generator.
This type of hybrid solar system could be used by utilities to generate grid electricity. That is the "vision". A lot of work needs to be done to get from today's laboratory setup to a production product in the field. A competitive product is probably 8 to 10 years away.
MIT - Can Solar Energy Be Economically Stored?
 Stored power has two major issues that have been holding back widespread acceptance of the technology: 1) the initial capital equipment cost is expensive relative to other alternatives, and 2) solar power is a daytime phenomenon. In the first case, the cost of PV solar is coming down rapidly and is expected to reach "grid parity" in a few years. In the second case, storage of solar power in batteries is very expensive and not enough capacity exists, the same as other alternatives. Daniel Nocera, Professor of Chemistry and Energy at MIT, and assistant, Matthew Kanan Postdoctoral Fellow, have made a scientific discovery that may lead to a cheap way to store solar energy in the future.Water is H2O - two hydrogen atoms and an oxygen atom. Scientists have known for a long time how to use electricity to split water into hydrogen and oxygen, which is called electrolysis. The discovery is a way of doing what existing electrolyzers do, but much more simply and efficiently. The key component in the process is a new catalyst which consists of cobalt metal, phosphate, and an electrode. Running a small electric current through the water, the water's oxygen atoms bubble up, leaving pure hydrogen in the water. Another separate catalyst recovers the hydrogen gas. Pure hydrogen, is an excellent way to store energy.
Stored power has two major issues that have been holding back widespread acceptance of the technology: 1) the initial capital equipment cost is expensive relative to other alternatives, and 2) solar power is a daytime phenomenon. In the first case, the cost of PV solar is coming down rapidly and is expected to reach "grid parity" in a few years. In the second case, storage of solar power in batteries is very expensive and not enough capacity exists, the same as other alternatives. Daniel Nocera, Professor of Chemistry and Energy at MIT, and assistant, Matthew Kanan Postdoctoral Fellow, have made a scientific discovery that may lead to a cheap way to store solar energy in the future.Water is H2O - two hydrogen atoms and an oxygen atom. Scientists have known for a long time how to use electricity to split water into hydrogen and oxygen, which is called electrolysis. The discovery is a way of doing what existing electrolyzers do, but much more simply and efficiently. The key component in the process is a new catalyst which consists of cobalt metal, phosphate, and an electrode. Running a small electric current through the water, the water's oxygen atoms bubble up, leaving pure hydrogen in the water. Another separate catalyst recovers the hydrogen gas. Pure hydrogen, is an excellent way to store energy. The oxygen and hydrogen gases can be stored in separate containers. At a later time, the oxygen and hydrogen gases can then be fed into a fuel cell, creating carbon-free electricity. (Fuel cells combine two gases, usually hydrogen and oxygen, to make electricity.) In the MIT lab, electricity came from the grid, but the electricity could come from solar panels, or for that matter, from wind turbines or hydropower. This system imitates the water-splitting reaction that occurs in nature which is called photosynthesis (carbon dioxide + water + light => stored sugar + waste oxygen).
 Dan Nocera's vision for the future is that each residential house will have a PV solar panel system on the roof. During the day the sun will generate electricity and the excess, that is not currently used for heat, air conditioning, etc., will be converted to hydrogen and oxygen gas using only ordinary water and stored in local tanks. When needed, the hydrogen and oxygen gas will be fed into a fuel cell to generate electricity at night or on cloudy days. The electricity could be used for home appliances or to charge an electric car or anything else. The fuel cell by-product is again water which would be fed back into the water tank. Most likely a connection to the grid would be maintained for backup purposes. This vision is probably ten-plus years away. Still, scientists need to have a road map to the future to guide them in their day-to-day activities along the way. Dr. Nocera has founded a new start-up company in the Boston area, Sun Catalytix Corporation, thanks to a $700,000 seed investment led by Polaris Ventures. That might sound like a small sum, but Nocera has been an epic center of buzz ever since he first published the research behind Sun Catalytix.
Dan Nocera's vision for the future is that each residential house will have a PV solar panel system on the roof. During the day the sun will generate electricity and the excess, that is not currently used for heat, air conditioning, etc., will be converted to hydrogen and oxygen gas using only ordinary water and stored in local tanks. When needed, the hydrogen and oxygen gas will be fed into a fuel cell to generate electricity at night or on cloudy days. The electricity could be used for home appliances or to charge an electric car or anything else. The fuel cell by-product is again water which would be fed back into the water tank. Most likely a connection to the grid would be maintained for backup purposes. This vision is probably ten-plus years away. Still, scientists need to have a road map to the future to guide them in their day-to-day activities along the way. Dr. Nocera has founded a new start-up company in the Boston area, Sun Catalytix Corporation, thanks to a $700,000 seed investment led by Polaris Ventures. That might sound like a small sum, but Nocera has been an epic center of buzz ever since he first published the research behind Sun Catalytix.There are still several major problems to be solved: Sun Catalytix needs to replace the platinum used in the "other electrode" in Nocera's hydrogen process with something cheaper. (Platinum is not cheap.) Also, the large-scale processes for manufacturing need to be worked out. Sun Catalytix recently received a contract for over $4 million in federal funding from the US Department of Energy (DOE). DOE selected Sun Catalytix as one of only 37 recipients from over 3,600 applicants for the first round of funding for transformational energy technologies. In October 2010 Sun Catalytix raised $9.7 million in a second round of financing led by Tata Limited.
There is competition, Nanoptek Corporation, also in the Boston suburbs, is pursuing its own catalytic, water-splitting process, and has raised $4.7 million in financing. For now, both companies are in stealth mode, refusing press inquiries. Their goals are obvious: A cheap way to get energy from the sun, just like plants do.
Space Based Solar Power (SBSP)
The ultimate solar power station is in outer space. As shown in the sketch at the far right, there are energy losses as the sun's rays are reflected from the atmosphere, clouds, dust, the day-night cycle and seasonal changes. Only about 50% of the sun's energy reaches the earth and then only 15% to 25% of that reaches the grid.
 As shown on the right-hand side of the sketch, a Space Based Solar Power (SBSP) system would collect solar energy by a satellite 24 hours a day, convert it to microwaves, and transmit the microwave radiation to Earth where it would be captured by a very large ground antenna and converted into usable electricity on the grid. The energy efficiency from the space station to the grid would be about 50%, roughly the same as fossil fuel electrical plants.
As shown on the right-hand side of the sketch, a Space Based Solar Power (SBSP) system would collect solar energy by a satellite 24 hours a day, convert it to microwaves, and transmit the microwave radiation to Earth where it would be captured by a very large ground antenna and converted into usable electricity on the grid. The energy efficiency from the space station to the grid would be about 50%, roughly the same as fossil fuel electrical plants.The SBSP concept was first published in November 1968 by Dr. Peter Glaser. In 1973 he was granted a U.S. patent for his method of transmitting power from a satellite using microwaves sent from a large antenna to a combination rectifier-antenna, now called a "rectenna" on the ground.
NASA began to study the concept in 1974. They found that the concept had several major problems - the huge expense of putting the solar satellite in orbit requiring hundreds of space trips and the lack of experience in space for projects of this scale. The proposal showed enough promise to merit further research. Research has continued to this day.
 There have been enormous improvements in solar panel efficiency. Research has continued to this day. There have been enormous improvements in solar panel efficiency reducing the size of the panel array in space. (See an artist's sketch of a proposed satellite system at the left.) Rocketry has also improved, but not sufficiently enough that "hundreds of transport trips" can be undertaken cost-effectively at this time. Satellite transportation costs remain the biggest obstacle to this technology being implemented. Many noted scientists believe that if the US "concentrated" on this effort, the costs of transporting the spacecraft materials to space would come down and the whole project would be cost-competitive with fossil fuels.
There have been enormous improvements in solar panel efficiency. Research has continued to this day. There have been enormous improvements in solar panel efficiency reducing the size of the panel array in space. (See an artist's sketch of a proposed satellite system at the left.) Rocketry has also improved, but not sufficiently enough that "hundreds of transport trips" can be undertaken cost-effectively at this time. Satellite transportation costs remain the biggest obstacle to this technology being implemented. Many noted scientists believe that if the US "concentrated" on this effort, the costs of transporting the spacecraft materials to space would come down and the whole project would be cost-competitive with fossil fuels.As we come to the later part of the 21st century and with the availability of fossil fuels declining resulting in extremely high fuel prices, many scientists believe SBSP will be seen as the ultimate answer. The real question is "Will we by then be ready to exploit this endless source of cheap electrical power?" For a more detailed discussion of SBSP see the Solar High Brochure by Dr. Philip Chapman former NASA Astronaut, Physics Professor at MIT, and Employee of Dr. Peter Glaser.
Ohio University has initiated a "SunSat Design Competition" which is "an international competition intended to accelerate the design, manufacture, launch, and operation of the next generation satellites that will collect energy in space and deliver it to earth as electricity. The Mission of the SunSat Design Initiative is to move space solar power out of the research labs and onto the public agenda. This will be done by virtual storytelling and networking on a global basis, explaining what space solar power is and how and why it will become the ultimate renewable energy resource for Planet Earth." See the Ohio University SunSat Design Competition.
Post a Comment:
You may also like:

Category
Featured Articles
Expanding Research on Solar Energy ...
The application fields of solar energy are very wide, covering many fields such as the photonic industry, new energy photothermal ...
Exploring Concentrated Solar Power ...
Concentrated Solar Power (CSP) systems use very different technology than photovoltaic systems. CSP systems use the sun as the ...
Solar Cell Manufacturing Process
Solar cells are made of various materials, the most common of which include silicon, indium gallium, cadmium selenide, etc. These ...
The Evolution of Grid Electricity ...
"Electricity" cannot be stored on the grid; generation must be approximately equal to consumption at all times. However, ...
Solar Activity: Sunspots, Magnetism & ...
Solar activity refers to a series of complex phenomena in the solar atmosphere, including sunspots, flares, prominences, coronal ...
